Magnetic Resonance-guided Focused Ultrasound (MRgFUS) is an advanced, incisionless treatment for essential tremor and tremor-dominant Parkinson’s disease. It uses concentrated ultrasound waves, guided by MRI imaging, to precisely ablate a tiny area in the brain that causes tremors – without open surgery or implants. This technique offers many patients rapid relief from tremors with minimal downtime. Below, we break down what patients and caregivers should know about MRgFUS, in accessible terms but with technical depth where useful.
Rapid Tremor Relief: How MRgFUS Works and Why Improvement Is Immediate
MRgFUS relieves tremors by creating a small lesion (tiny burn) in the brain region responsible for the involuntary shaking. For essential tremor and Parkinson’s tremor, the target is usually a spot in the thalamus called the VIM nucleus, which acts like a “relay station” for tremor signals (advances.massgeneral.org). During the procedure, hundreds of ultrasound beams pass harmlessly through the skull and converge at the VIM, heating it to disrupt the abnormal tremor signals (advances.massgeneral.org).
This process is done gradually and carefully. At first, low-energy ultrasound pulses are delivered while the patient is awake and feedback is obtained – the medical team asks if the patient feels any tingling or other sensations and checks whether the tremor is improving (greenwichhospital.org). Because the patient remains awake, they can perform simple tasks (like drawing a spiral or touching their nose) between each pulse so doctors can see in real time if the tremor is lessening (advances.massgeneral.org)(wakehealth.edu). The energy is increased stepwise until the tremor noticeably subsides without side effects, and only then is a higher dose given to create a permanent lesion in that spot (advances.massgeneral.org).
Immediate improvement is one of the most remarkable aspects of MRgFUS. Patients often experience a dramatic reduction in tremors during the procedure itself or immediately after the final lesion is made. In fact, doctors report that “most patients will experience immediate and often dramatic improvement in their tremors immediately after the procedure” (advances.massgeneral.org). Many individuals who could not hold a cup or write legibly due to tremor are able to do so right after MRgFUS. For example, one patient with a 40-year history of tremors had tears of joy as she fed herself and drank water without shakiness immediately after treatment (greenwichhospital.org)(greenwichhospital.org). On average, studies find about a 50–75% tremor reduction in the treated hand right away (barrowneuro.org). This rapid relief is possible because MRgFUS directly targets the brain circuit causing the tremor – once that circuit is disrupted, the abnormal shaking often stops or greatly diminishes on the spot.
Incisionless and Non-Invasive: No Scalpels, Implants, or General Anesthesia
Unlike traditional brain surgeries, MRgFUS does not require any incisions in the scalp or skull. There is no need to open the skull or insert electrodes into the brain. Instead, the ultrasound transducer (a helmet-like device) is positioned on the patient’s head and delivers sound waves through the intact skin and bone. Because it is incisionless, MRgFUS carries no risk of wound infection and leaves no surgical scar (pulse.cedars-sinai.org). Patients also avoid having any foreign hardware implanted in their body – a key difference from deep brain stimulation (DBS) surgery, which leaves wires and a pacemaker-like device under the skin.
MRgFUS is typically done without general anesthesia. Patients remain awake and communicative throughout the treatment, needing only a localized numbing of the scalp (for a head frame placement) and possibly a mild sedative to relax (uclahealth.org). There’s no ventilator or breathing tube as used in asleep surgeries. This makes the procedure far less taxing on the body, especially for older patients. In fact, doctors note that for elderly patients or those on blood thinners who are high-risk for open surgery, focused ultrasound is much safer – “with focused ultrasound, there’s no blood work or anesthesia and little risk of infection”, and after the one-time treatment patients go home the same day with improved tremor (pulse.cedars-sinai.org).
Because it doesn’t involve cutting or hardware placement, MRgFUS is usually performed as an outpatient procedure. Patients check in, undergo the treatment, and can often leave later that day once recovered from any sedative. No hospital stay is required in most cases (barrowneuro.org). The lack of incision also means recovery is quick – there’s no surgical wound to heal. Aside from some temporary sensations (addressed later), most people resume light activities within a day or two. In summary, MRgFUS offers the tremor relief of a surgical procedure without actual surgery, making it a compelling option for those who cannot or do not want to undergo traditional brain surgery (advances.massgeneral.org).
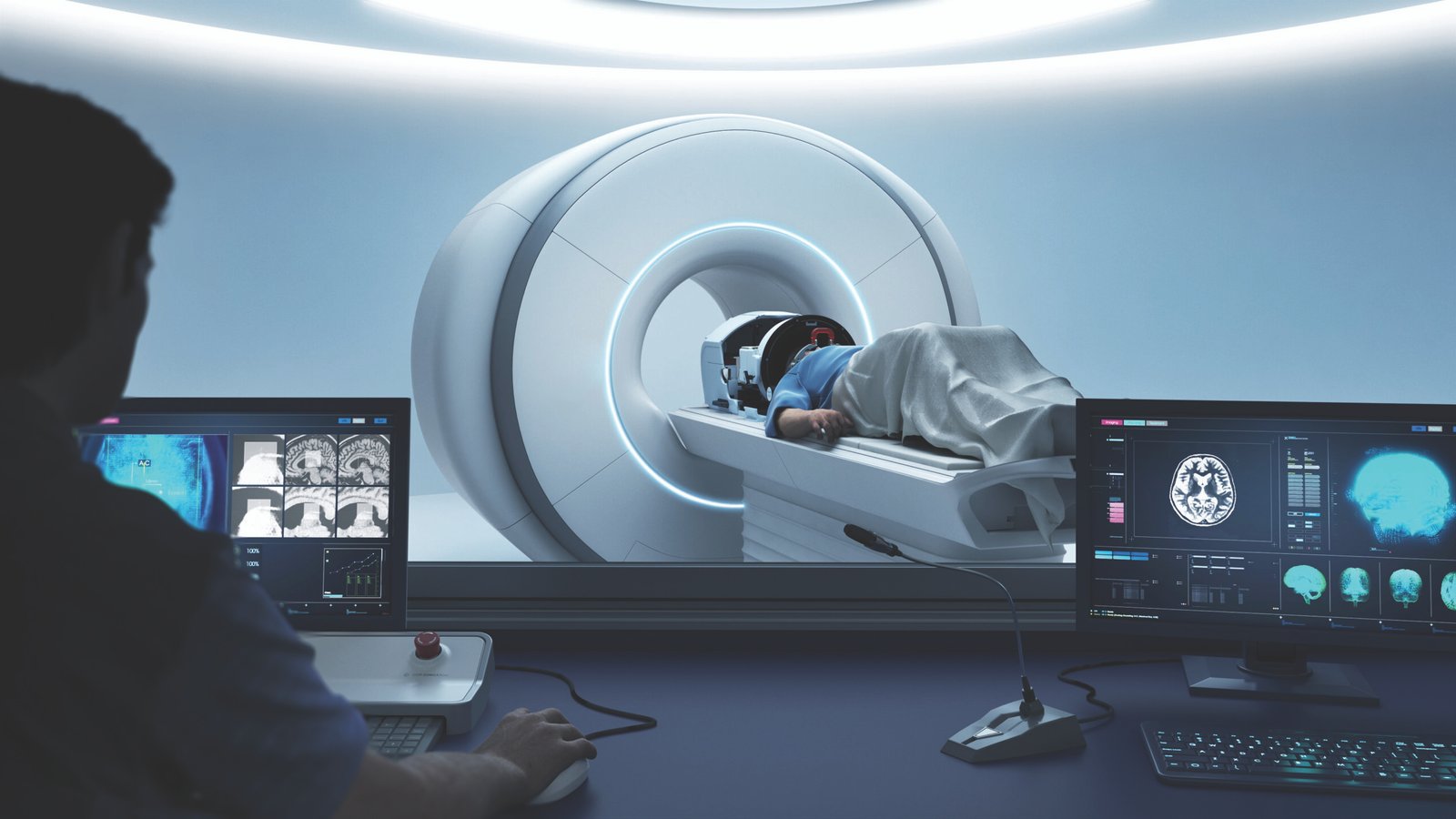
MRI Guidance for Precision: Targeting Tremor Cells with Millimeter Accuracy
An MRI scanner plays a critical role in the focused ultrasound procedure. In essence, the MRI serves as the “eyes” of the treatment team (uclahealth.org). The patient lies in an MRI machine throughout the procedure, which provides continuous high-resolution images of the brain. These images allow neurosurgeons to pinpoint the exact area of the brain that needs treatment and to ensure the ultrasound energy is hitting only that spot.
Before treatment, an MRI (and often a CT scan of the skull) is used to map the patient’s unique anatomy and plan the target coordinates (uclahealth.org). During the procedure, MRI helps in two main ways: navigation and safety. First, MRI guidance ensures the focused ultrasound waves are aligned to converge precisely on the VIM nucleus (or other intended target). A special rigid head frame is attached to the patient’s skull to keep the head perfectly still and to provide a 3D reference for targeting (uclahealth.org). This frame, coupled with computer guidance and MRI, lets doctors aim the ultrasound beams with sub-millimeter accuracy at the tiny tremor-causing region.
Second, MRI provides real-time feedback during the ultrasound delivery. MRgFUS systems use MRI-based thermometry – essentially, MRI scans can measure temperature changes in the brain tissue. The medical team can literally see where and how much the tissue is heating up (advances.massgeneral.org). This feedback is crucial: it confirms the energy is concentrated in the right place and not overheating surrounding areas. If the patient feels any side effect or the MRI shows off-target heating, the team can immediately adjust the focus before making a permanent lesion (greenwichhospital.org). They incrementally increase power under MRI monitoring until the tremor improves, then finalize the treatment. This level of precision would be impossible without MRI guidance. In earlier eras, surgeons performed “blind” thalamotomies with only crude measurements, or had to insert electrodes to test the location. With MRgFUS, by contrast, the combination of MRI imaging and computer guidance ensures the treatment is delivered to the exact spot responsible for tremor while sparing other brain tissue (greenwichhospital.org). The result is a very focused therapy – hence the name focused ultrasound – with a lower risk of affecting other functions.
Who is Eligible for MRgFUS, and Who Is Not?
Ideal candidates for MRgFUS are patients with either essential tremor or Parkinson’s disease in whom tremor is the primary, disabling symptom. Typically, candidates have moderate to severe tremor that significantly impacts their quality of life (for example, trouble eating, drinking, writing, or working due to tremor) (advances.massgeneral.org). Both conditions (ET or PD) should be diagnosed by a neurologist or movement disorder specialist – it’s important to be sure the tremor is from these diseases and not another cause. Doctors also generally require that the tremor has not responded adequately to medication. This means patients should have tried the standard tremor-suppressing drugs (for ET, usually propranolol and primidone; for Parkinson’s, medications like levodopa or others) and either gotten little relief or had intolerable side effects (advances.massgeneral.org) (barrowneuro.org). One source notes candidates must have failed at least two appropriate medications for their tremor disorder (pulse.cedars-sinai.org). Essentially, MRgFUS is considered when medications can’t control the tremor and it is impacting daily living.
Age requirements: In the U.S., MRgFUS for tremor is FDA-approved for adults age 22 or older (pulse.cedars-sinai.org). There is no strict upper age limit – even patients in their 80s have been treated – as long as they are in reasonable health. In fact, some older patients who aren’t good candidates for invasive surgery (DBS) due to age or medical issues may qualify for MRgFUS. Every patient will undergo a thorough evaluation to ensure they can tolerate lying in an MRI for a few hours and that they don’t have conditions that would make the procedure unsafe (uclahealth.org).
Key eligibility factors and typical criteria: All of the following are usually required for MRgFUS:
- Diagnosis of ET or tremor-dominant Parkinson’s: Other movement disorders like dystonia are not yet approved for this treatment. (Research is ongoing, but currently only ET and PD tremor are treated (wakehealth.edu.)
- Significant, disabling tremor in one arm/hand: Often the focus is on the dominant hand. Tremor should be severe enough to warrant a surgical-level intervention (often a physician will quantify tremor severity on a scale). Mild tremors that don’t interfere with daily tasks may not justify the risks of MRgFUS.
- Tried and not benefited from medications: As noted, at least two standard medications should have been attempted without sufficient success (pulse.cedars-sinai.org). MRgFUS is usually only offered for medication-refractory tremors.
- One side is more affected: MRgFUS is typically performed on only one side of the brain at a time (to improve the tremor in the opposite hand). Patients whose tremor is markedly worse on one side (e.g. the right hand shakes more than the left) are ideal, because treating the worst side will give the most benefit (pulse.cedars-sinai.org). (If both sides are equally severe, bilateral treatment is possible but done in two stages – discussed later.)
- Able to undergo MRI and lie still: The patient must be able to have an MRI (no incompatible metal implants, and no severe claustrophobia that can’t be managed). Metal in the body that is magnetic or interferes with MRI can disqualify a patient – for example, certain aneurysm clips or older pacemakers that are not MRI-safe (uclahealth.org). Likewise, one must be able to lie flat, with the head fixed in a frame, inside the MRI scanner for about 2–3 hours. Patients who cannot lie still that long due to back pain or other issues may have difficulty with the procedure (uclahealth.org).
- Skull characteristics: An important and little-known eligibility factor is the thickness and density of the skull bone. Ultrasound must pass through the skull; if someone’s skull is too thick or dense, the ultrasound may not effectively reach the brain. A specialized CT scan is done before approval specifically to analyze the skull – if the “skull density ratio” is too low, the patient might not be a candidate for MRgFUS (uclahealth.org). This can exclude a small percentage of patients.
- General health: While MRgFUS is less invasive than surgery, patients should still be in reasonably stable health. Conditions like uncontrolled high blood pressure, recent heart attack, severe kidney disease (e.g. on dialysis), or active infection could postpone or contraindicate the procedure (uclahealth.org). Each center will review a patient’s overall medical status. Notably, because no general anesthesia is used, many patients with chronic conditions can still safely undergo MRgFUS if those conditions are managed.
Who is NOT a candidate? Aside from not meeting the above criteria, there are specific exclusions. Patients who have certain implanted devices may be ineligible – for instance, a patient who already has a deep brain stimulator or a vagus nerve stimulator might not be able to have MRgFUS due to the metal implants and the fact that MRgFUS is often considered an alternative to having hardware. (Someone who previously had DBS but didn’t get good results would typically need the DBS hardware removed before considering MRgFUS (barrowneuro.org.) Individuals with metallic implants in the body that cannot be safely brought into an MRI (like some cochlear implants, older pacemakers, metal fragments in eyes, etc.) are usually excluded (uclahealth.org).
Patients with severe tremor in both hands may not get both sides treated at once – simultaneous bilateral MRgFUS is not done because of safety concerns (doing lesions on both sides of a certain brain nucleus can cause speech or balance problems). Such patients either undergo staged treatment (one side first, the other 6–12 months later) or are steered toward DBS, which can treat both sides with one surgery (pulse.cedars-sinai.org). Also, if tremor is not the dominant problem – for example, a person with Parkinson’s whose main issues are walking and stiffness rather than tremor – MRgFUS would not be appropriate because it addresses tremor circuits specifically.
Other relative contraindications include significant pre-existing balance or neurological issues. Because MRgFUS can cause temporary balance impairment, someone who already has severe gait instability or neuropathy might be advised to consider alternatives (advances.massgeneral.org). Extensive scar tissue on the scalp (from burns or prior surgeries) could interfere with the ultrasound beams and cooling system (uclahealth.org). And as mentioned, patients who simply cannot tolerate being still and awake in an MRI for hours (due to anxiety or pain) might not be suitable.
Each center offering MRgFUS typically has a checklist. As an example, Barrow Neurological Institute suggests you may be a good candidate if you have ET or tremor-dominant PD, failed at least 2 medications, have a dominant-hand tremor that’s moderate-to-severe, and are not a DBS candidate due to advanced age, medical comorbidities or personal preference (barrowneuro.org). You may not be a good candidate if you have health issues that make MRI or steady positioning impossible, or if your tremor is mild enough to be managed otherwise(barrowneuro.org). Ultimately, an interdisciplinary team (neurologist, neurosurgeon, etc.) will evaluate each patient to decide if MRgFUS is appropriate (wakehealth.edu). If someone isn’t eligible, that team will discuss other options that suit the patient’s situation.
How Long Do the Effects Last?
MRgFUS thalamotomy is intended as a one-time, permanent treatment for tremor. The lesion created in the tremor circuit of the brain does not “wear off” – it’s a lasting change, similar to the surgical thalamotomies performed in past decades. In many patients, the tremor reduction achieved is long-lasting, and they enjoy improved motor function for years afterward. However, it’s important to set realistic expectations: essential tremor and Parkinson’s are progressive neurological conditions, meaning the underlying disease can continue to worsen with time. So while the treated tremor is often much better, some patients may experience a gradual return of tremor symptoms over the years (advances.massgeneral.org).
What do studies show? The initial clinical trial of MRgFUS for essential tremor found that tremor improvement at 12 months was nearly as good as right after treatment (pubmed.ncbi.nlm.nih.gov). Patients’ tremor ratings improved significantly and that benefit was largely maintained at one year. Beyond one year, longer-term follow-ups indicate that most patients retain a substantial tremor reduction for at least 3–5 years or more. A five-year study reported that the hand tremor severity in treated patients was still 73% better than baseline at 5 years post-treatment (pulse.cedars-sinai.org). Similarly, other reports have shown improvements persisting at 3 years and even up to 6 years after the procedure (rush.edu) (uclahealth.org). In one series, the range of improvement from baseline at 3 years was about 50% in tremor severity, showing that while there might be some waning compared to immediately post-treatment, the tremor was still much improved relative to before treatment (rush.edu).
Crucially, these outcomes assume no other treatment is done in between – the MRgFUS lesion itself is providing the benefit long-term. For many, the effect is life-changing: they gain years of steadier hands. That said, doctors caution that a minority of patients do experience some recurrence or worsening of tremor over time (advances.massgeneral.org). Why would tremor come back if the lesion is permanent? There are a few possibilities:
- Progression of disease: Essential tremor can slowly get worse with age, potentially involving wider tremor networks. Parkinson’s disease is neurodegenerative, so other symptoms and even tremor can progress. The MRgFUS treats one target spot, but it doesn’t halt the disease process, so new tremor pathways might emerge or the untreated side may worsen over time.
- Conservative initial treatment: During MRgFUS, the team balances maximum tremor control with safety. They may intentionally make a smaller or less intense lesion if the patient starts to get side effects. This “conservative” approach avoids complications but could leave a bit of residual tremor. Sometimes immediately after the procedure, the tremor is greatly improved but not 100% gone. That residual tremor could become more noticeable if the disease progresses, or if the brain “rewires” a little around the lesion.
- Lesion changes: Generally the lesion (a tiny scar) is permanent, but very rarely, the effect could diminish if the lesion was small. (This is unlike DBS where if you turn off the stimulator the tremor returns instantly – with FUS, there’s no on/off, it’s a fixed change.)
The good news is that even when tremor does creep back to a degree, it usually does not return to full pre-treatment severity. Many patients years later are still better off than they were before MRgFUS, even if not quite as dramatically as right after treatment. In one study, 63% of patients had sustained improvement at last follow-up (meaning some did have their tremor increase again, but the majority maintained significant benefit) (pubmed.ncbi.nlm.nih.gov). Doctors will monitor patients over time with follow-up visits and tremor ratings. Some patients might resume or adjust medications if slight tremor returns, to supplement the procedure’s benefit.
Repeat Procedures: Because MRgFUS creates a lesion, it’s not something that is routinely “repeated” on the same side. Unlike an adjustable DBS pacemaker, if tremor returns significantly on the treated side, doing another focused ultrasound treatment in that same area is generally not done (the tissue is already lesioned once, and safety of a second lesion is uncertain). However, there is the possibility of treating the other side if needed. Initially, MRgFUS was only approved for one side of the brain (usually treating the dominant hand’s tremor). More recently, based on clinical data, staged bilateral treatments are being allowed.
In 2023, the FDA approved MRgFUS for treating the second side in essential tremor patients who already had one side done, with a recommended minimum interval of 9 months between sides (fusfoundation.org). This means if a patient had their right-hand tremor fixed and later the left hand’s tremor becomes disabling, they can undergo MRgFUS on the left side as a separate procedure. Studies of this staged approach show that doing the second side after waiting (often 9–12 months) can further improve bilateral tremor control while keeping side effects manageable (pulse.cedars-sinai.org).
For Parkinson’s disease, the situation is similar. MRgFUS is typically done on one side to help the side of the body with worse tremor. The effect on tremor is long-lasting, but Parkinson’s disease may progress in other ways. Researchers are evaluating FUS for treating both sides or other symptoms of PD, but as of now, if other symptoms (like rigidity or balance) worsen, additional treatments or medications might be needed. The tremor relief from the thalamotomy should remain, though.
In summary, patients can expect immediate and often years-long tremor relief from a single MRgFUS treatment. Most will have enduring benefit; for some, tremor may slowly increase again due to the nature of their condition. Importantly, MRgFUS doesn’t “cure” the underlying disease, but it does provide sustained symptomatic relief. If tremor does significantly relapse on the treated side (which is uncommon in the short term), doctors would re-evaluate and could consider other options (like DBS or in the future perhaps a second lesion, though that’s not standard currently). For untreated tremor on the opposite side, a second MRgFUS can be planned after an interval if appropriate. Overall, the durability of MRgFUS’s effect has been one of its strong points – data up to 5 years are very encouraging, indicating it’s not just a temporary fix (pulse.cedars-sinai.org).
Side Effects and Risks: What to Expect
Although MRgFUS avoids the risks of open surgery, it still carries some risks because it creates a small lesion in the brain. Patients do not experience any pain from the lesion itself (the brain doesn’t feel pain), but they might feel sensations during the procedure like warmth or tingling. The key is that most side effects are temporary and mild, but a few patients may have longer-lasting issues. Here we explain potential side effects in simple terms:
Common short-term side effects: It’s normal to have some transient symptoms right after the procedure. Many patients feel imbalance or unsteady on their feet immediately after treatment (barrowneuro.org). You’ve essentially caused a tiny disruption in the brain, and the areas around it can become temporarily irritated or swollen, which might affect balance or coordination for a short time. Patients often describe it as feeling a bit off-balance or as if they’re tipsy when walking. Because of this, centers usually recommend using a walker or cane initially and caution against driving for a short period (barrowneuro.org)(barrowneuro.org). This unsteadiness is usually temporary – in fact, many people’s balance improves within days or weeks as the brain adjusts and any swelling subsides.
Another common short-term effect is numbness or tingling, often in the face, lips, or fingers on the treated side (pubmed.ncbi.nlm.nih.gov)(barrowneuro.org). For example, a patient treated for right-hand tremor might notice their right fingertips feel a bit numb or their lip on one side tingles. This happens because the tremor circuit is near sensory pathways. In the initial trial, about 38% of patients had some numbness/tingling afterward, but importantly it was mostly mild and transient (pubmed.ncbi.nlm.nih.gov). Other short-run side effects can include headache, dizziness, or nausea on the day of the procedure (barrowneuro.org) (barrowneuro.org). These might result from being in the MRI or slight irritation of the brain. They typically resolve within hours to days and can be managed with medications if needed (a short course of steroids is sometimes given to reduce any swelling, which helps these symptoms resolve faster (barrowneuro.org).
Temporary gait disturbance: As noted, balance issues are common short-term. Cedars-Sinai specialists report that “many people do experience a transient sense of gait instability following treatment that can last up to three months… but it almost always improves, and by one year out, balance typically returns to normal.”(pulse.cedars-sinai.org). This aligns with trial data: about one-third of patients had some gait disturbance right after MRgFUS, but only about 9% still felt any balance issues a year later (pubmed.ncbi.nlm.nih.gov). In other words, most balance side effects are temporary and the small fraction that persist are usually mild.
Less common or rare risks: In a few cases, side effects can be more pronounced or longer-lasting. The clinical trial found that at 12 months, about 14% of patients had some persistent numbness/tingling and 9% had some persistent gait difficulty (pubmed.ncbi.nlm.nih.gov). These were generally mild (for instance, slight fingertip numbness that doesn’t really impair function, or a mild balance issue). However, there is a small risk of more significant or permanent neurological deficits – for example, muscle weakness on one side, loss of coordination, or speech difficulties. These are uncommon, but because the thalamus is near pathways controlling movement and speech, an imprecise lesion or unexpected reaction could potentially cause such issues. In practice, the careful, MRI-guided approach is designed to avoid this, and serious complications have been quite rare. No long-term cognitive impairment has been linked to unilateral thalamotomy (the procedure is not near memory or thinking centers).
It’s worth noting that doing lesions on both sides of the thalamus historically carried a risk of affecting speech and cognitive functions. This is why if both sides are needed, MRgFUS is done in two stages with a long gap and careful evaluation in between (pulse.cedars-sinai.org). By keeping treatment to one side at a time, those major risks are minimized.
Other considerations: Unlike open surgery, MRgFUS doesn’t have bleeding or infection risks from a wound. Nonetheless, there is a minor risk of skin burns or damage if the scalp isn’t cooled properly (the system uses a water cooling helmet and monitors to prevent this). Modern systems have safety checks to avoid overheating the skull or skin. Some patients may get a mild scalp irritation or redness from the headframe or the ultrasound, but this is typically minor and heals quickly. The pin sites from the head frame (usually four small points on the scalp) may be sore or bruised for a few days, similar to having had a tight clamp – these spots are bandaged and heal without issue (barrowneuro.org). There is also the general consideration of no guarantee of effect: a small number of patients might not get the desired tremor relief even if the procedure is technically successful (or they might get initial benefit that doesn’t meet their hopes). In the initial trial, a few patients did not have significant tremor improvement despite treatment (and some in the sham group improved due to placebo). However, the vast majority in treatment groups do improve substantially(pubmed.ncbi.nlm.nih.gov).
Plain language summary of side effects: Right after MRgFUS, you might feel wobbly on your feet or have some tingling – don’t panic, this is common and usually goes away. You should plan to take it easy for a few days. Most patients say these sensations fade over days or weeks. By three months out, the large majority of people feel normal in terms of balance and touch (pulse.cedars-sinai.org). Only a small percentage have any lasting symptoms, and these are usually minor (for example, a little numbness in a finger). Doctors will discuss all these risks with you beforehand. It’s important to weigh that even if there is, say, a 10-15% chance of a mild lasting side effect, there is also a very high chance of significantly reducing your tremor which may greatly improve your quality of life. In the published trial, the researchers concluded that “MRI-guided focused ultrasound thalamotomy reduced hand tremor in patients with essential tremor. Side effects included sensory and gait disturbances.”(pubmed.ncbi.nlm.nih.gov) – essentially, it works, but can cause some numbness or walking difficulty in some patients. Understanding the balance of these outcomes will help you make an informed decision.
Clinicians mitigate risks by performing the procedure slowly with feedback (so if something starts to feel off, they can stop before permanent effects occur) (greenwichhospital.org). If a patient already has trouble with balance or sensation, they will consider that carefully before recommending MRgFUS (advances.massgeneral.org). In practice, many patients are willing to accept the small risk of side effects given the prospect of tremor relief. And importantly, MRgFUS does not close the door to other treatments – if needed, one could still have DBS surgery later on (though typically MRgFUS is done as an alternative to DBS).
To date, serious complications like hemorrhage or life-threatening events have been exceedingly rare with MRgFUS, especially when done at experienced centers. It’s a well-tolerated procedure for most. Doctors will monitor you closely after treatment – you may have a follow-up MRI or exam to ensure everything is okay. If any side effects linger, rehab therapies (like physical therapy for balance or occupational therapy for any hand numbness) can often help.
The Role of Advanced Neuroimaging: How Imaging Improves Accuracy & Safety
Neuroimaging is the cornerstone of MRgFUS – without imaging, this treatment wouldn’t be possible. Beyond just guiding the ultrasound during the procedure (as discussed earlier), imaging is used at every step to enhance safety and effectiveness:
- Patient Selection and Planning: Before MRgFUS, patients typically get a high-resolution MRI of the brain and often a CT scan of the skull. These images let doctors visualize the exact anatomy – they can locate the VIM nucleus on the MRI and measure the skull thickness on CT. This planning is crucial to determine if the focus can be reached and to calibrate the device settings for each patient (uclahealth.org). If imaging shows any unexpected abnormalities (like an old stroke or tumor near the target), the team can plan around them or decide if it’s too risky. Thus, imaging helps screen for potential issues and tailor the treatment plan to the individual’s brain structure.
- Precise Target Identification: The VIM is a tiny region, only a few millimeters across. MRI allows neurosurgeons to identify this region either directly or via anatomical landmarks. Some centers even integrate functional imaging or tractography to see connections, ensuring that they hit the spot that halts the tremor and avoid critical nearby tracts. This level of precision was not possible decades ago – it’s the advanced MRI capabilities that let us lesion a target that’s deep inside the brain without open surgery. One neurosurgeon explained that MRgFUS is a “high-tech, safer, and more precise implementation” of a well-understood surgery (thalamotomy)(advances.massgeneral.org)– it’s the precision of modern imaging that makes it so.
- Real-Time Monitoring: As described, MRI thermometry maps temperature changes in real time. Think of it like having a live thermometer and GPS inside the brain during the treatment. Doctors can watch a color-coded map that shows how hot the target spot is getting. This ensures they deliver enough heat to make the lesion (around 55–60°C at the target) but not so much that heat bleeds into unwanted areas. If the imaging showed heat in an unintended area, they would pause or adjust immediately. This capability greatly improves safety – it’s a big reason MRgFUS can claim precise targeting without collateral damage (advances.massgeneral.org)
- Verification of Results: After the full dose is delivered, MRI is used to verify that the lesion is in the correct place and the tremor circuit is effectively disrupted. The team often takes a post-treatment MRI to see the small lesion formed. In many cases, they’ll do another neurological exam (still inside the MRI room) to double-check tremor improvement on the spot. The imaging combined with clinical testing confirms success. If the tremor is not adequately improved and imaging suggests the lesion was slightly off, theoretically they could do another sonication to adjust – but this is uncommon if planning was done well. Essentially, imaging confirms “we hit the bullseye.”
- Post-Procedural Imaging: Some time after the procedure (hours or a day later, or at follow-ups), MRI may be done again to see how the lesion and surrounding brain look. This can rule out any swelling or edema that might need treatment (for example, if there was unexpected swelling, steroids can be given to reduce it). It also documents the precise location and size of the lesion for the patient’s records. Later MRIs can show if the lesion has shrunk or changed (typically it becomes a tiny scar spot). This is useful for research and for any future care the patient might need.
In summary, neuroimaging enhances both accuracy and safety by guiding the therapy to the right spot, monitoring it in real time, and avoiding damage to healthy brain tissue. It transforms a procedure that would otherwise be like “shooting in the dark” into one that is as controlled as possible. One patient-friendly explanation is that the MRI is like a GPS and thermal camera combined, allowing surgeons to “see” exactly where to treat and how the tissue responds, which dramatically lowers the risk of mistakes (uclahealth.org). Advanced MRI sequences and computer software also help in co-registering the patient’s CT and MRI, calibrating for skull characteristics, and focusing the beams. In effect, MRgFUS harnesses imaging technology to do surgery without a scalpel – the images replace the surgeon’s direct line of sight and hands.
As imaging technology continues to improve (for example, higher-power MRI machines or better real-time resolution), we can expect MRgFUS to become even more precise. Some research is looking at using other imaging feedback, like ultrasound imaging or advanced MR sequences, to possibly guide treatment for other brain targets as well. But for now, high-quality MRI is indispensable for MR-guided focused ultrasound.
Insurance Coverage and Costs: What to Expect
Navigating insurance and costs for a cutting-edge treatment like MRgFUS can be challenging, and it varies by region. Here’s an overview of how coverage and costs break down in different regions:
United States: In the U.S., MRgFUS for essential tremor has been FDA-approved since 2016 and for tremor-dominant Parkinson’s since 2018 (for unilateral treatment). Medicare – the federal health insurance for seniors and disabled – now covers MRgFUS for medication-refractory essential tremor in all 50 states (fusfoundation.org). Medicare coverage falls under Part B (outpatient procedure) (insightec.com). This was a significant development, as it means most patients over 65 can get the treatment with Medicare paying the bulk of the cost (patients would be responsible for any copay or deductible like usual). Private insurers have been gradually following suit. Many major private insurance companies (Aetna, Blue Cross/Blue Shield, Cigna, etc.) do cover MRgFUS for essential tremor when certain criteria are met (barrowneuro.org). Typically, the patient must have tried and not found relief from at least two tremor medications, and the request often needs prior authorization. For Parkinson’s tremor, insurance coverage is more limited – as of 2023, Medicare covers it (since it’s FDA approved), but fewer private plans automatically do (barrowneuro.org). Some patients with PD tremor may need to appeal or get case-by-case approval from their insurer since it’s a newer indication.
For those paying out-of-pocket (uninsured or if the procedure isn’t covered), the cost in the U.S. is significant. Estimates range roughly from $35,000 to $50,000 USD for the entire treatment, including hospital fees, use of the MRI suite, and the specialist team(israelihospitals.org.il). This ballpark can vary by center; some top hospitals might charge more, and some research institutions might charge less. $40k is a common rough figure cited for self-pay. However, with Medicare and insurance coverage increasingly available, many patients in the U.S. will not have to pay the full amount. Instead, they’ll pay their normal insurance cost-sharing (for example, a Medicare patient might pay 20% after meeting their deductible, unless they have supplemental insurance). Tip: It’s always wise to confirm coverage with your insurance beforehand. Provide them the CPT code 0398T (the billing code for MRgFUS on tremor) and ask if it’s covered (insightec.com). Many centers also have nurse navigators who can help patients navigate insurance approval.
Gulf Countries (Middle East): In the Gulf region – which includes countries like the United Arab Emirates, Saudi Arabia, Qatar, Kuwait, Bahrain, Oman – MRgFUS is relatively new but available in some advanced medical centers. For example, the UAE has offered MRgFUS at cutting-edge hospitals (such as Cleveland Clinic Abu Dhabi) for a few years. Coverage in Gulf countries varies. In nations with government-funded healthcare for citizens (like Saudi Arabia or Qatar), the state may cover the cost if the treatment is deemed necessary and is available locally. Some Gulf residents also travel abroad for MRgFUS under government sponsorship if it’s not yet offered widely at home. Private insurance in the Gulf may cover it if the policy is comprehensive and the treatment is available in-country. However, if paying out-of-pocket in the Gulf region or as a medical tourist, the cost is comparable to Western countries. A clinic in Dubai quotes prices around $20,000 USD and up for MRgFUS treatment. Realistically, total costs could be higher once hospital stay (if any), consultation, etc., are included. Patients from Gulf countries often choose to travel to centers of excellence (in Europe or the US) if it’s not yet established at home, sometimes at personal expense or with government funding. The Gulf region’s adoption of MRgFUS is growing, with conferences and experts in the region reporting successful treatments for tremor in recent years. It’s advisable for patients in Gulf countries to check with major neurology/neurosurgery centers in their country to see if MRgFUS is offered locally and how payment works (public vs private). In summary, Gulf residents can access MRgFUS, but they should expect high costs if paying themselves (roughly on the order of $20k–$30k). If local public healthcare covers it, the personal cost may be minimal, but availability might be limited to certain hospitals.
Western Europe: In Europe, MRgFUS is approved (the device has CE marking) and is available in multiple countries, though access can vary. Generally, Western European countries with national health services are beginning to cover MRgFUS for essential tremor on a case-by-case or center-of-excellence basis. For instance, in the UK, the National Health Service (NHS) has approved focused ultrasound for essential tremor – it’s offered at a few specialist centers and is NHS-funded for eligible patients (parkinsons.org.uk). Similarly, countries like Germany, Switzerland, Spain, France, Italy, and Portugal have medical centers performing MRgFUS, often in university hospitals or specialized clinics. In Germany, focused ultrasound for ET has received reimbursement approval under the NUB system (a mechanism for new treatments), which means insurance (statutory health insurance) can cover it in hospital settings. Norway and the Nordic countries, known for advanced healthcare, have also evaluated MRgFUS; Norway’s public health system has been looking into offering it, which likely would be covered for citizens much like other hospital treatments (once available) (nyemetoder.no). Austria has an active program (for example, in Vienna), and Spain and Italy have multiple sites, often with public hospital access. Ireland and Luxembourg, being smaller countries, as of the mid-2020s, might not yet have their own MRgFUS center (Ireland’s health system has focused on establishing DBS surgery; MRgFUS may be in planning). Patients there could be referred abroad, perhaps to the UK or continental Europe, for treatment. In the EU, there’s a system of cross-border healthcare where a patient in one member state can receive treatment in another and seek reimbursement, especially if the treatment isn’t available in their home country.
For those who pursue private treatment in Europe or pay out-of-pocket, costs are a bit lower than U.S. prices but still substantial. The procedure in Western Europe typically costs about €20,000 to €30,000 EUR (approximately $21,000–$32,000 USD) in total(israelihospitals.org.il). For example, centers in Germany or Switzerland have quoted in this range. This usually includes the hospital fees and any brief stay needed. Some countries may offer it a bit cheaper if done in a research context or in public hospitals with subsidized costs. Also, some European insurance plans (statutory or private) might cover MRgFUS fully, leaving patients with little or no out-of-pocket expense, depending on the country’s health system. For instance, in countries with universal healthcare, if MRgFUS is approved as a standard treatment, patients might pay only normal hospital copayments or nothing at all. Norway and Luxembourg likely fall into this category once they have the service: the national insurance would cover it as it does for DBS, given appropriate referrals. Ireland may require special approval or sending the patient to a UK center until it’s locally available.
In summary, European patients should consult their neurologist about local options. If MRgFUS is offered in-country, there’s a good chance it can be covered by the public system or insurance after evaluation, especially for essential tremor. If seeking private treatment, expect costs in the tens of thousands of euros, albeit somewhat lower than U.S. list prices (israelihospitals.org.il). Western Europe as a whole is embracing MRgFUS, with the UK already providing it on the NHS for ET (parkinsons.org.uk), and other countries not far behind in making it accessible.
A note on other regions: In Israel, which is a leader in this technology (the company Insightec is based there), the cost is around $30,000–$45,000 USD privately (israelihospitals.org.il), and the treatment is available at major centers. Some patients from countries without access travel to Israel for treatment. In Asia, interestingly, costs can be much lower in certain countries – for example, in India, some hospitals offer focused ultrasound for around $10,000 USD (israelihospitals.org.il), thanks to lower hospital fees, and in Japan and Korea there are national programs treating patients (with coverage in some cases). Availability is spreading globally, but insurance coverage always lags a bit behind technology. The Focused Ultrasound Foundation keeps a list of treatment sites worldwide, which can be a resource (israelihospitals.org.il).
Bottom line on costs: MRgFUS is an expensive high-tech procedure. In the U.S. and Europe, it runs in the five-figure range. However, insurance and national health programs are increasingly covering it for those who need it, especially for essential tremor. Patients should engage with their healthcare providers and insurers to determine coverage. If insurance is not an option, discuss payment plans or financial assistance the hospital might offer; some hospitals have charity care or research trials that can defray costs. Given the significant improvement in quality of life many experience, efforts are underway to make this treatment more widely covered by health systems around the world.
Preparing for the Procedure: What Patients Should Do Before MRgFUS
Proper preparation can help the MRgFUS treatment day go smoothly and safely. Once you’ve been deemed an eligible candidate and scheduled for the procedure, your medical team will give you specific instructions. Here’s generally what to expect and how to prepare:
- Pre-Treatment Evaluations: You will likely undergo a few appointments before the actual procedure day. This includes meeting with the neurologist and neurosurgeon to review everything, a possible neuropsychological assessment (to establish cognitive baseline), and importantly, imaging tests – typically an MRI of the brain and a CT of the head. The CT is done to check your skull’s properties and help with treatment planning (wakehealth.edu)(wakehealth.edu). These steps don’t require special prep on your part besides showing up.
- Medication Adjustments: Your doctors may adjust certain medications before the procedure. For example, if you are on blood thinners (like warfarin, clopidogrel, or novel anticoagulants), they might ask you to pause them for a few days prior, because even though there’s no incision, the head frame pins and the potential for minor bleeding or bruising exist. (And generally, interventions are safer with normal clotting.) Always do this in consultation with your doctor – do not stop medications on your own. If you take tremor medications (like propranolol or primidone), the team might instruct you to hold them the night before treatment (neurosurgicalva.com). This is so that your natural tremor is apparent during the procedure for testing – if the meds are masking tremor somewhat, it’s harder to gauge improvement. On the other hand, some centers allow a light dose of certain meds – follow the instructions given by your team.
- The Night Before: Typically, you’ll be asked not to eat or drink after a certain time (often midnight) before the day of the procedure. This is a common precaution in case sedation is used (even though it’s not general anesthesia, an empty stomach is safer with any sedative). They may allow small sips of water for essential pills. You should also wash your hair and scalp thoroughly the night before, because your head will be shaved and prepped in the morning – starting with a clean scalp reduces infection risk and helps the ultrasound transmission. Get a good night’s rest as you’ll be at the hospital for much of the next day.
- What to Bring: Bring a form of ID and your insurance info as usual, and any required paperwork the hospital gave you. Wear comfortable clothing; you’ll change into a hospital gown for the procedure, but loose clothes and slip-on shoes for after are nice. You won’t be able to wear any metal in the MRI, so leave jewelry at home. Also, arrange for a responsible adult to accompany you to the hospital – this person will drive you home or accompany you afterward. You will not be allowed to drive yourself home (due to sedation and potential imbalance). Plan for someone to stay with you the first night just to be safe. Consider bringing a walking aid (cane or walker) if you have any concerns about balance after – many centers suggest this as a precaution (barrowneuro.org).
- Day of Procedure – Before Treatment: When you arrive, you’ll check in and go through standard pre-op vitals. You will likely have an IV placed (for fluids or medication during the procedure). The first big step is head shaving – be prepared: the entire scalp must be shaved to skin (uclahealth.org)(wakehealth.edu). Ultrasound does not pass well through hair, and a clean scalp is needed for the cooling helmet to work effectively. This can be emotionally daunting for some, but hair will grow back, and many patients say it’s a small trade-off for steady hands (wakehealth.edu). Next, the team will attach the stereotactic head frame to your skull. This sounds intense, but it’s done under local anesthesia at the pin sites. You’ll get numbing injections on the forehead and back of head where a few pins will secure the frame (uclahealth.org)(wakehealth.edu). The frame is like a rigid ring that prevents your head from moving even a millimeter during the treatment (essential for accuracy). You might feel pressure as it’s applied, but it shouldn’t be painful after the numbing. Once the frame is on, they may do a quick CT or x-ray with it to assist in targeting setup.You’ll be given a light sedative and pain medication if needed to keep you relaxed and comfortable, but you will remain awake and able to converse (uclahealth.org). Some centers actually use no sedation, just a mild tranquilizer – the goal is for you to be alert enough to respond and perform tasks. Don’t hesitate to speak up if you feel anxiety – they can give a bit more sedative as long as you stay responsive.
- During the Procedure: You’ll be positioned on the MRI table and slid into the scanner with the head frame locked in place. A special helmet device will be secured around your head; this helmet has dozens of ultrasound emitters and circulates cool water around your scalp to keep it at a safe temperature (wakehealth.edu). You might feel the coolness, but it prevents your skin from heating. The team will take some initial MRI scans for planning – you’ll just need to lie still. They will be in constant communication via microphone (“intercom”); you’ll hear them and can talk back – there’s usually even a panic button or stop button you can press if you need a break (wakehealth.edu).The treatment consists of multiple sonications (ultrasound pulses). For each one, you’ll hear the MRI humming (since MRI is scanning simultaneously) and possibly hear a buzzing or feel a slight sensation when the ultrasound is delivered. The first few sonications are low-power: you might feel nothing or you might feel a slight warmth or tingle in your head or arm/face. After each sonication, they will slide you out of the MRI just enough to do an exam. You’ll be asked to perform some simple tasks, like drawing a spiral on a clipboard, touching your finger to your nose, moving your hand, or speaking, depending on what they’re checking(wakehealth.edu). They are looking to see if your tremor improved and if you have any side effects at that dose. You and the doctors are essentially testing the effect in real time. It’s actually a fascinating process – you might notice your hand is already steadier after a test dose, which can be very encouraging mid-procedure.If all is well (tremor improving, no bad side effects), they will increase the energy a bit and repeat. You’ll go in and out of the MRI multiple times. As it progresses, you might start to feel some warmth in your body or head – that’s normal, the team monitors it (uclahealth.org). The cold water helmet usually keeps you comfortable, but if you feel too warm or any pain/heat on the scalp, tell them immediately (they can pause). You may also hear a popping sound or feel a little pop in your head at higher sonications – patients have described hearing a “knocking” or feeling a tick – this is the ultrasound effect and is generally not painful, but just an odd sensation.
- After the Treatment: Once the team is confident that the tremor is controlled and the lesion is made, the active part is over. The actual sonication time might be a total of a few minutes, but because of all the pauses and imaging, the whole procedure usually lasts around 2–3 hours on the table (barrowneuro.org)(wakehealth.edu). You will then come out of the MRI, and the frame will be removed (that part is quick – you’ll just feel a release of pressure and maybe a brief pinch). They will clean the pin sites and apply small bandages. You’ll be kept for observation for a couple of hours in the recovery area (uclahealth.org). The medical team will do a more thorough neurological exam, and you’ll likely get to experience using your hand without the tremor – for instance, they might have you write your name or drink from a cup to see the difference. This is often a joyous moment for patients and staff alike.During recovery, as the mild sedative wears off, you might start to notice some of the temporary side effects described earlier (slight dizziness, unsteady gait). Nurses will monitor your blood pressure, etc., but generally, recovery from MRgFUS is straightforward. Many patients can go home later that same day (uclahealth.org). Make sure your companion is present to hear discharge instructions. You might be given a short course of steroids (like a prednisone pack) to take for a few days to minimize any swelling in the brain (barrowneuro.org). You could also get a prescription for something like meclizine (for dizziness) or a mild dexamethasone (steroid).
- Going Home and Aftercare: Have your helper drive you home. You should plan to rest the remainder of the day. You can eat normally as tolerated (some may feel a little nausea from the experience, but many are fine and even eager to celebrate a bit). Keep the pin site bandages on until the next day; you can usually remove them and shower about 24 hours after, gently washing the scalp (barrowneuro.org). Your head will be shaven – feel free to wear a hat or scarf if you go out; new hair will start growing soon.You’ll likely notice immediate tremor improvement in the treated hand – enjoy those first moments of doing things steadily! But also remember to be cautious with walking until any imbalance fades. Use handrails, and don’t rush into activities like ladders or carrying very hot liquids until you’re confident in your balance. Many centers advise not driving for a short period (a few days up to two weeks) until you’re sure you have no significant side effects and your reaction time is normal (barrowneuro.org). Follow-up appointments will be scheduled, often at 1 month and 3 months, to check on your progress.
In summary, preparing for MRgFUS mostly involves following instructions such as medication adjustments, fasting if needed, and mental preparation for having your head shaved and being awake during a several-hour procedure. The medical team will do most of the heavy lifting in terms of planning and intraoperative work. Patients should focus on arranging support (a ride and possibly someone to stay with them), and on understanding the steps so they feel comfortable. Many patients find that knowing what to expect – for example, that they will be awake, and that it’s normal to feel some funny sensations – greatly reduces anxiety. Don’t hesitate to ask your doctors any questions before the procedure. On the day, you can typically listen to music through special MRI-compatible headphones if the facility offers it, or even converse with the team between sonications – whatever keeps you calm.
One patient noted that the procedure was long but “not painful” and that the moment she could draw a steady spiral during the treatment was incredibly exciting (greenwichhospital.org). By being well-prepared and following guidelines, you set yourself up for the best possible outcome and a smooth recovery.
The Future of MRgFUS and Non-Invasive Neurosurgery: Innovations on the Horizon
MRgFUS is part of a broader trend toward incision-free neurosurgery, and its success in treating tremor is just the beginning. Researchers and clinicians are actively exploring new ways to use focused ultrasound and improving the technology further. Here are some promising developments and future applications:
- Expansion to Other Neurological Conditions: The principle of using focused ultrasound to lesion or modulate brain circuits could help treat other disorders. One example is Obsessive-Compulsive Disorder (OCD). Traditionally, severe OCD has been treated with lesions in certain brain areas (a procedure called cingulotomy or capsulotomy). Now, focused ultrasound is being tested to create those lesions without open surgery (pulse.cedars-sinai.org). Early trials are underway to see if MRgFUS can safely alleviate OCD symptoms by targeting the relevant circuits. Another area of interest is depression and other psychiatric illnesses that might benefit from precisely targeted lesions or neuromodulation – though this is in very early stages.
- Treating More Parkinson’s Symptoms: So far, MRgFUS in Parkinson’s is mostly used for tremor (targeting the thalamus). However, Parkinson’s disease has other motor symptoms like rigidity and dyskinesia (involuntary movements from long-term medication use). Researchers have been investigating MRgFUS pallidotomy – targeting the globus pallidus (another deep brain structure) – to address these symptoms. Small trials have shown that lesioning the globus pallidus with focused ultrasound can reduce dyskinesias and improve motor function in Parkinson’s pubmed.ncbi.nlm.nih.gov. A large international trial for MRgFUS pallidotomy was conducted, and the results are encouraging, suggesting it could become an option for patients with medication-induced dyskinesias or medication-refractory Parkinson’s motor symptoms (pubmed.ncbi.nlm.nih.gov). In the future, we might see MRgFUS used to treat not just tremor-dominant PD, but also other forms of Parkinson’s by targeting different brain regions – all without incisions.
- Pain Disorders: Chronic neuropathic pain or certain facial pain syndromes could potentially be treated by MRgFUS lesioning of pain pathways. There have been studies using focused ultrasound to treat trigeminal neuralgia (a severe facial pain condition) or other pain by targeting specific thalamic nuclei or other structures. This is analogous to procedures like cordotomy or thalamotomy for pain done surgically in the past, now being explored with FUS.
- Epilepsy: Although still very experimental, there is interest in using focused ultrasound to create tiny lesions in epileptic foci (areas of the brain that trigger seizures) or to modulate those areas. Focused ultrasound can also open the blood-brain barrier (discussed below), which might help deliver anti-seizure drugs to specific regions. MRgFUS might one day provide a non-invasive alternative to procedures like hippocampal ablation for drug-resistant epilepsy.
- Blood-Brain Barrier (BBB) Opening for Drug Delivery: A revolutionary use of focused ultrasound, at lower power settings, is temporarily opening the blood-brain barrier to help medications reach the brain. The blood-brain barrier often blocks large therapeutic molecules (like certain chemotherapy drugs or antibodies for Alzheimer’s). By using microbubbles and ultrasound, researchers can transiently open the barrier in a targeted spot. This has been tried in conditions like Alzheimer’s disease – to help clear amyloid plaques or deliver drugs – and brain tumors, to enhance chemotherapy delivery (pulse.cedars-sinai.org). Focused ultrasound in this context does not destroy tissue; it just makes the brain blood vessels briefly more permeable. Early-phase studies have shown it’s possible and safe to repeat periodically. This could herald treatments where, for example, a patient with Alzheimer’s gets focused ultrasound sessions to allow therapeutic antibodies to enter the brain more effectively.
- Non-Invasive Neuromodulation: Apart from permanently lesioning tissue, focused ultrasound at very low energies can actually stimulate or inhibit neurons non-destructively. This is being researched as a way to neuromodulate brain circuits on demand, similar to how transcranial magnetic stimulation (TMS) works, but potentially with deeper reach. For instance, a focused ultrasound wave (below the level that causes heat) can transiently excite or silence a region. This could be used for temporary therapeutic effects or brain mapping. While not yet in clinical use, this concept might expand the toolkit for treating depression, OCD, or even as a functional brain mapping tool in neurosurgery.
- Technological Improvements: Future iterations of MRgFUS machines may be more powerful or more efficient. Researchers are working on higher-frequency ultrasound that can create even smaller lesions with finer control. Improved MRI guidance (like 3-Tesla or 7-Tesla MRI units with sharper imaging) could enhance targeting. There’s also work on reducing treatment time – perhaps multi-focus sonications that can shorten the procedure. The helmet arrays might be refined to better handle various skull types, potentially expanding eligibility (so fewer patients are excluded due to skull density issues). One can imagine next-gen systems that even adjust for patient motion or physiologic brain movement in real time, making the targeting even more exact.
- Bilateral and Combined Treatments: As experience grows, doing bilateral treatments safely could become more routine, as already seen by FDA’s approval for staged bilateral ET treatment (fusfoundation.org). Ongoing trials in treating both sides for Parkinson’s (for example, for people with severe tremor on both sides or other symptoms requiring bilateral action) will inform guidelines on how to do it with minimal risk (fusfoundation.org). Additionally, MRgFUS might be combined with other therapies – for example, using a small FUS lesion plus deep brain stimulation, or FUS plus gene therapy (opening BBB to deliver gene vectors, etc.). The concept of “combo therapy” is being considered in some experimental designs.
- Other organ systems: While our focus is the brain, it’s worth noting that focused ultrasound is also being used non-invasively in other parts of the body – from treating uterine fibroids to prostate tumors and beyond. The success in tremor has energized research in other neuro applications, like using FUS to treat brain tumors (by directly ablating a tumor or improving drug delivery to it)(pulse.cedars-sinai.org). There’s even exploration of ultrasound for stroke (helping break clots or disrupt the blood-brain barrier to deliver clot-busting drugs faster) and for neuromodulation in psychiatric disorders as mentioned.
In the big picture, MRgFUS is changing the face of neurosurgical medicine (pulse.cedars-sinai.org). Twenty years ago, the idea of performing brain surgery with sound waves and no incision seemed like science fiction; today it’s a reality for tremor patients, and tomorrow it could be reality for many others. As one neurosurgeon put it, this technology is “imminently expandable” – meaning we are just at the start of what it can do (pulse.cedars-sinai.org). The hope is that more and more patients will have access to effective treatments without the trauma of open surgery. For patients and caregivers, this means less risk, less downtime, and potentially wider availability (since you don’t need an operating room – just an MRI suite and the device).
Ongoing research and trials will dictate the pace of new approvals. It’s an exciting field to watch. In the near future, we might see MRgFUS being offered for conditions like chronic pain, depression, epilepsy, or aggressive psychiatric disorders, pending trial results. We’ll also likely see improvements in the user experience – perhaps shorter procedures, maybe not even requiring the rigid head frame if motion compensation tech improves (making it even more comfortable).
For essential tremor and Parkinson’s tremor, the future holds the possibility of treating more patients (including those who need both hands treated) and refining the technique to further minimize side effects. Because MRgFUS can be repeated for the other side or potentially other targets, it provides a flexible platform for lifelong disease management without repeat surgeries.
In conclusion, MRgFUS has already established itself as a cutting-edge, patient-friendly therapy for tremor. It offers rapid relief, minimal invasiveness, and lasting benefits for many. With ongoing innovations, it stands at the forefront of non-invasive neurosurgery – a field that aims to make treatments as safe and gentle as possible while still effectively treating serious brain conditions. The success in tremor is paving the way for a future where brain disorders can be treated with ever less disruption to the patient’s life, truly embodying the idea of “surgery without scalpels.” (pulse.cedars-sinai.org)
Ready to Explore Your Options for Tremor Relief?

If you or a loved one are dealing with tremor and want to learn more about your specific treatment options, we’re here to help.
Our dedicated coordinators offer a free personal consultation to discuss access to MRgFUS and other therapies. Additionally, our movement disorder specialists are happy to review your medical files—at no cost—to provide an informed, professional opinion on your case. Reach out to us today to get started on the journey toward improved quality of life.
References (Sources):
- Schweitzer, J. – Mass General Hospital, on MRgFUS for tremor: “Most patients will experience immediate and often dramatic improvement in their tremors immediately after the procedure.” (Q&A) advances.massgeneral.org
- Mass General Hospital – Explanation of how focused ultrasound targets the VIM thalamus with gradual energy increases, using MRI thermometry to guide treatment until tremor is suppressed without side effects advances.massgeneral.org.
- Mass General Hospital – Notes that MRgFUS requires “no incision, no devices are placed into the brain, and there is no need for general anesthesia or surgical incisions.” (Contrast with DBS) advances.massgeneral.org.
- Cedars-Sinai (Pulse Newsletter) – Dr. Patil: “For older patients, or for those unable to stop taking blood thinners, surgery may not be possible. With focused ultrasound, there’s no blood work or anesthesia and little risk of infection. After a one-time treatment, patients go home the same day with their tremor better controlled.” pulse.cedars-sinai.org
- Cedars-Sinai – 5-year follow-up: “A recent article in the Journal of Neurosurgery confirms the benefits of MRgFUS, showing sustained and significant tremor improvement after five years… tremor for the treated hand were improved by more than 73% from baseline at 60 months.”pulse.cedars-sinai.org
- Cedars-Sinai – Eligibility: “Candidates for MRgFUS must be at least 22 years old, have a confirmed diagnosis of ET or tremor-dominant Parkinson’s, and be unresponsive to or ineligible for at least two standard-of-care medications.”pulse.cedars-sinai.org
- Cedars-Sinai – Bilateral treatment: must wait minimum 9 months between sides, due to cognitive risk if done too soon pulse.cedars-sinai.org.
- Cedars-Sinai – Transient side effects: “Many people do experience a transient sense of gait instability… up to three months… by one year out, balance typically returns to normal.” pulse.cedars-sinai.org
- Cedars-Sinai – Future applications: OCD lesioning being tested; studies using MRgFUS to deliver targeted chemotherapies for brain tumors pulse.cedars-sinai.org.
- UCLA Health – Patient info: Medicare Part B covers MRgFUS for essential tremor uclahealth.org insightec.com; procedure overview (CT for skull, frame placement with mild sedative, shaved scalp, MRI helmet with cooling, patient awake giving feedback)uclahealth.org uclahealth.org.
- UCLA Health – “The entire procedure is conducted inside an MRI scanner which functions as the eyes of the treatment. MRI provides images to plan, treat and verify complete ablation of target.”uclahealth.org
- UCLA Health – Outcomes: “On average, tremor improves by ~50% and effects are sustained in most people up to 6 years after treatment. Side effects include risks of numbness or tingling or walking difficulty, which are rarely serious or disabling.”uclahealth.org
- Greenwich Hospital/Yale New Haven Health – Patient story: “The noninvasive procedure targets and disrupts tremor-causing brain tissue with precision. Under MRI guidance, sound waves are focused on the small spot… Low energy is first applied… patient is examined for tremors after each step… The energy is gradually increased to create a small therapeutic lesion. Many patients… experience immediate improvement.” greenwichhospital.org greenwichhospital.org
- Greenwich Hospital – After treatment: patient could feed herself and write without tremors, with “remarkable improvement” in handwriting immediately aftergreenwichhospital.org.
- NEJM (Elias et al. 2016) – Pivotal trial results: “MRI-guided focused ultrasound thalamotomy reduced hand tremor in patients with essential tremor. Side effects included sensory and gait disturbances.” pubmed.ncbi.nlm.nih.gov
- NEJM trial – Adverse events: gait disturbance in 36% (persisting in 9% at 12 mo), paresthesia/numbness in 38% (persisting in 14% at 12 mo) pubmed.ncbi.nlm.nih.gov.
- Barrow Neurological Institute – Patient FAQ: Benefits: “Focused ultrasound does not require an incision… performed as an outpatient… immediate improvement in tremor; on average 50–75% improvement.” barrowneuro.org
- Barrow Neurological Institute – Possible complications: lists temporary or permanent muscle weakness, unsteadiness, numbness/tingling, nausea or headache as potential effects barrowneuro.org.
- Barrow Neurological Institute – Insurance (Arizona example): Medicare, Medicare Advantage, Aetna, BCBS, Cigna cover ET; for PD tremor, mainly Medicare/Advantage cover barrowneuro.org barrowneuro.org.
- Barrow Neurological Institute – Candidate criteria: ET or tremor-dominant PD diagnosis; tried ≥2 meds; dominant hand tremor moderate-severe; not a DBS candidate due to age/comorbidities, or failed DBS barrowneuro.org.
- Focused Ultrasound Foundation – Medicare coverage: “Eligible essential tremor (ET) patients can now access reimbursement of focused ultrasound treatment through CMS in all 50 states.” (As of July 2020) fusfoundation.org
- Israeli Hospitals Association – Cost estimates: USA: $35,000–$50,000 (including pre/post care) israelihospitals.org.il; Europe: €20,000–€30,000 (≈$21k–$32k) israelihospitals.org.il ; UAE (via Bookimed): starting around $20,000 us-uk.bookimed.com.
- Parkinson’s UK – “In the UK, focused ultrasound is already approved to treat essential tremor on the NHS… by targeting and destroying cells in an area of the brain that controls movement.” parkinsons.org.uk
- Focused Ultrasound Foundation – FDA approval for second-side (bilateral) ET treatment (2023): patients can have the other side treated ≥9 months after the first, due to new ruling fusfoundation.org fusfoundation.org.